Research - (2022) Volume 5, Issue 4
Hesitancy Dilemma Regarding Covid19 Vaccination
Shaymaa Hussein Rafat Kotb*Received: Dec 05, 2022, Manuscript No. AMDHS-22-82248; Editor assigned: Dec 07, 2022, Pre QC No. AMDHS-22-82248 (PQ); Reviewed: Dec 10, 2022, QC No. AMDHS-22-82248 (Q); Revised: Dec 11, 2022, Manuscript No. AMDHS-22-82248 (R); Published: Dec 24, 2022, DOI: 10.5530/amdhs.2022.4.12
Abstract
COVID-19 is a respiratory disease caused by the SARS-COV-2 virus which is responsible for the high mortality rate in the last 3 years. The covid-19 crisis changes the world's vision of infectious diseases. Vaccination is the master key in protective control behavior for COVID-19. Anti-SARS-CoV-2 vaccines are the safest and most effective modality to stop the COVID-19 pandemic which limits the hospital and intensive care unit admission. World Health Organization delay acceptance or refusal of vaccines and considered it a critical decision-making procedure. Although the high safety profile of the vaccine, there were reported rare adverse events following vaccine administration. Global adverse events are categorized into; uncommon, common, and very common. The vaccine induces the immune-mediated response which takes days to develop, so resulting a lifelong immunity or a subsequent exaggerated adverse reaction in the second booster dose. The aim of this study is to thorough light on the importance of vaccines to limit the wide spread of the virus and raise awareness of physicians toward different common and rare adverse reactions to promote early diagnosis and so prevent complications.
Keywords
COVID-19, Vaccination, Vaccine hesitancy, Vaccine adverse events, SARS-CoV-2
Introduction
COVID-19 (Coronavirus Disease 2019), is one of the most widespread and significant public health crises of recent decades due to the development of Severe Acute Respiratory Syndrome (SARS-CoV-2). The World Health Organization considered it a pandemic disease. It is spread rapidly and requires hospitalization or even intensive care and with every infection comes new opportunities for the virus to mutate. Genomic mutations are anticipated events during virus replication, and although most mutations are expected to be neutral, some can confer a fitness advantage and be fixed in the viral genome. During the last two years of the pandemic, the coronavirus is responsible for more than five and a half million confirmed deaths worldwide. COVID-19 symptoms include fever or chills, cough, shortness of breath, and loss of smell and taste. WHO recommended accordingly, non-pharmaceutical prevention interventions include face masks, social distancing, ventilation of indoor spaces, hand hygiene, and Vaccines [1].
The fear of being infected with SARS-CoV-2 has become widespread, especially among older adults. Vaccination is a key for the protective behavior against COVID-19 which plays an important role in increasing population immunity, preventing serious diseases, and reducing the health crisis. Vaccination and immunization remain the best options for the prevention and control of diseases worldwide. World Health Organization (WHO) recommended vaccines for emergency use, including four types of COVID-19 vaccines: mRNA, inactivated virus, viral vector-based, and recombinant protein vaccine. Vaccination significantly reduced the risks of COVID-19 infection, hospitalization, admission to the intensive care unit, and death. Since the development of a vaccine, scientists uses the spike protein as an antigen, and the mutations of the SARS-CoV-2 in the spike protein make the efficacy of the vaccine a concern. Some studies have examined the protection of vaccinations against variants in a population. The results showed that the vaccine is still effective against the current variants, but its effectiveness is weakened in the infected patients. The mutation of the virus makes the prevention situation serious, and changes in vaccine strategies will continue to be monitored [2].
The anxiety attitude regarding vaccination is particularly amplified by systematic media messages focusing on incidence and mortality. The need to maintain social distance, which involves limiting interpersonal contact, is another factor predisposing to increased perceived anxiety. Information campaigns to promote mass vaccination against COVID-19 are a key element in controlling and preventing the spread of the pandemic. However, the success of such health education measures depends primarily on vaccination coverage in a given population. Older people were the priority group for vaccination worldwide and the first to receive basic doses as well as booster doses due to their higher risk of developing severe disease if infected [3].
When COVID-19 Virus invades the body elicits an immune response, which results in fever. This process greatly speeds up the adhesion and migration of immune cells to lymph nodes and tissues at the site of infection. As phagocytes engulf the virus, they also release cytokines, which recruit additional immune cells to participate. Neutrophils are a major component of the leukocyte population capable of releasing large amounts of reactive oxygen species and pro-inflammatory cytokines, and lymphocytes are an important part of the adaptive immune response against the virus. Meanwhile, helper T cells stimulate B cells to produce specific antibodies that bind to the virus and prevent it from entering the cell, allowing it to aggregate and then be engulfed by phagocytes. If the immune system wins such a battle against the virus, the body recovers and preserves its immunity to SARS-CoV-2, and the B cells can proliferate rapidly and produce a large number of antibodies when re-infected. IgG is the primary antibody against bacteria, antivirus, and antitoxin in serum [4].
Following vaccination antigenic stimulation-induced, the development of long-term protective immunity depends on a range of immunological events, including the activation, proliferation, differentiation, and coordination of the humoral and T-cell response. The vaccine can elicit a coordinated spike-specific T-cell response characterized by the production of all Th1 cytokines, with IFN correlating with both TNF_ and IL2. Cellular immunity after COVID-19 vaccination probably plays a contributing role in controlling SARS-CoV-2 infection, even in the absence of a humoral response [5].
The development of an adaptive immune response, encompassing neutralizing antibodies and B-lymphocytes and T-lymphocytes, is important to controlling and clearing viral infections. It has been reported that individuals infected with SARS-CoV-2 develop an antibody titer around 15 days after the onset of the symptoms. It is well-known that the antibody titer diminishes over time. The presence of increased IgGs was observed with the first dose in participants with primary infection. Although antibodies are a central component of vaccine efficacy, B-lymphocytes are fundamental to the adaptive immune system. B-lymphocytes are also associated with an increase in pro-inflammatory cytokines, such as IL-6. It is important to enhance response to the TLR9 and may function to switch B-lymphocyte responsiveness from adaptive BCR-mediated signaling to innate TLR-receptor signaling. Neutralizing Antibodies (nAbs) are more indicative of protective immunity due to their ability not only to bind S-RBD but also to block viral entry to the host cells. Therefore, neutralization assays remain the gold standard for measuring the nAbs titer against SARS-CoV-2 [6-8].
Although vaccines are the best tool available to hinder the progress of the COVID-19 pandemic. They were highly safe and effective but sometimes potentially associated with adverse events. The use of the term Adverse Events Following Immunization (AEFI) has spread to include “any adverse medical event that follows vaccination. The World Health Organization (WHO) and Council for International Organizations of Medical Sciences (CIOMS), propose classifying AEFI into five groups:
1. Reactions related to the vaccine product
2. Reactions related to defects in the quality of the vaccine
3. Reactions related to immunization errors
4. Reactions related to anxiety
5. Coincident events
On the other hand, the WHO published a Manual for Causality Assessment of AEFI, which includes four possibilities of immunization reactions: (A) Consistent with causal association to immunization, (B) Indeterminate, (C) Inconsistent with causal association to immunization, and (D) Unclassifiable [9 ].
Vaccine hesitancy according to the World Health Organization (WHO) is the delay in acceptance, reluctance, or refusal of vaccination despite the availability of vaccination services. It is one of the top 10 threats to global health in 2019 because it is a complex decision-making process. Vaccine hesitancy results from a complex interrelation of behavioral and societal factors. This is vaccine hesitancy or acceptance depends on multiple factors such as (i) Contextual factors: Lower monthly household income; (ii) Individual factors: No intention of taking the flu vaccine this year, perceiving their health status as reasonable and having two or more diseases; (iii) COVID-19 influences: Low confidence in the health service response to COVID-19 and non-COVID-19, worse perception of the adequacy of measures implemented by the government, perceiving a low or non-existent risk of getting COVID-19, and feeling agitated, anxious or sad some days due to the physical distance measures; and (iv) COVID19-vaccine-specific factors: lack of trust in the safety and efficacy of the vaccines [10].
Vaccine hesitancy results from The “Three Cs” model of vaccine Confidence, Complacency, and Convenience defined by McDonald and collaborators is one of the most useful given that it is intuitive and easy to understand and apply. The confidence which known as the degree of trust in the efficacy and safety of the vaccine, in the system that delivers the vaccines, and in the motivations of those who make the decisions to achieve effective access to the vaccines. Lack of confidence can be influenced by misinformation about vaccination risks, affiliation to anti-vaccine groups, or legitimate concerns regarding vaccine safety and efficacy and trust in government and the pharmaceutical industry. Complacency is defined as the perception that the risk of diseases preventable by vaccination is low, and when vaccination is not considered a necessary or the chief preventive measure. Complacency is affected by the level of knowledge of diseases and vaccines as well as by prejudices regarding vaccines. Convenience is defined as the influence on vaccine acceptance of vaccine availability, affordability, willingness to pay, ability to understand and accept vaccine-related information, health service quality, and the degree to which vaccination services are delivered at a time and place and in a cultural context that is convenient and comfortable [11].
Vaccine hesitancy is a multidimensional phenomenon associated with one’s attitudes and beliefs, in addition to environmental, social, cultural, and political factors. Many factors may influence parents’ willingness to vaccinate against COVID-19, social media and wrong information from inaccurate sources may be the major ones. Global adverse events following COVID-19 vaccination vary based on the type of vaccines, These heterogenous adverse events were reported in three categories: uncommon, common, and very common. The most common symptoms reported include fatigue, headache, muscle and joint pain, allergic skin reaction, and chills, while the most prevalent events include low-grade fever and pain or redness at the site of injection, often felt a few days after vaccination. Severe adverse events are possible, but the chances are low [12].
Vaccine hesitancy is a major barrier to achieving herd immunity in different populations. The most common reasons for COVID-19 vaccine hesitancy among the population were concerned with the adverse effects Generally, vaccines may produce adverse reactions due to idiosyncrasies or because of the body’s immunological system. despite the identification of serious and fatal adverse events following COVID-19 vaccination, a causal relationship has not been established. There were reports of people who developed Thrombosis with Thrombocytopenia Syndrome (TTS) after getting the Johnson & Johnson (J&J) vaccine. Also, reports of myocarditis and, pericarditis related to Pfizer-BioNTech and Moderna COVID-19 vaccines [13].
Although some of the adverse events were reported to resolve within a few days after vaccination they may be the reactions of the immune system shortly after vaccination. The CDC recommends that individuals having severe allergic reactions immediately (within 4 hours) or some days after administration of the vaccine should refrain from getting a second shot of the type of vaccine that produced the event. After administrated of the COVID-19 vaccine, there is a transient local inflammation signaling neutrophil and antigen-presenting elicited at the site of injection. According to the CDC, following the administration of the first dose of the COVID-19 vaccine, if an itch or swollen or painful rash is observed in a person, said person(s) should be treated with antihistamine or acetaminophen. If fatigue or pain is observed, treatment is equally recommended before such candidates proceed for the second shot of the vaccine based on availability to affirm complete protection [14].
Vaccines facing some challenges, one of them is Shoulder Injuries Related to Vaccine Administration (SIRVA), the preferred medicolegal term since 2017 for an Adverse Event Following Immunization (AEFI) affecting the shoulder musculoskeletal region, which is an uncommon and poorly understood consequence of improper vaccination administration. It is causally linked to improper vaccination technique or location and is considered to be preventable by the Australian Immunization Handbook through anatomical land marking techniques. Raise awareness of SIRVA to increase the ability to recognize, diagnose, manage, and report suspected cases, which is considered of concern to all healthcare Practitioners [15].
Moreover, vaccinations can prevent the downstream effects of COVID-19 infectious diseases, They show susceptibility to the occurrence of adverse reactions. Possible causes of an allergic reaction to a COVID-19 vaccine are usually due to the reaction to adjuvants and other excipients or components in the vaccine, rather than to the active principle itself. All allergic reactions are immune-mediated, but not all immune-mediated reactions are allergic. Vaccination associated as a possible trigger for autoimmune and auto-inflammatory, or mixed disease phenotype [16].
There were some reports of patients presenting an autoimmune or autoinflammatory disease after the application of the COVID-19 vaccine. A syndrome similar to Kawasaki disease was reported for the first time in association with the vaccine. Kawasaki Disease (KD) is an acute multisystemic vasculitis, manifested by a collection of signs and symptoms, including fever, polymorphic rash, conjunctival injection, cervical lymphadenopathy, changes in the oral mucosa such as erythema and cracking of the lips, strawberry tongue, diffuse injection of oral and pharyngeal mucosa, and characteristic changes in the extremities, such as desquamation, erythema, and edema of the palms and soles. KD is an exaggerated inflammatory response to environmental triggers in genetically susceptible individuals, that stimulate a large number of T lymphocytes. These superantigens can interact directly with the major histocompatibility complex class II molecules on the surface of T cells and induce the following modifications: Release of cytokines, activation of B cells and mononuclear cells, and adhesion of inflammatory cells to the endothelium leading to vasculitis [17].
Eosinophilic Granulomatosis with Polyangiitis (EGPA) is another allergic immune-mediated disease that reported adverse reactions to appear following the administration of the vaccine. It is a small-vessel vasculitis characterized by asthma, eosinophilia, and eosinophil-rich granulomatous inflammation in various tissues. Respiratory tract involvement is almost manifestation present, and the peripheral nervous system, gastrointestinal tract, and myocardium are also commonly affected. The patient’s history was remarkable for rhinoconjunctivitis, mild asthma, and allergic sensitization to house dust mites, cat dander, and grass [18].
Another reported case of association between the vaccine and Bell’s palsy and Guillain-Barre syndrome. polyneuritis cranialis is multiple cranial neuropathies in the absence of weakness of the limbs. It is reported that neurological or infectious disease presented with numbness and drooping on the right side of her face, with no history of adverse reactions to any drug or vaccine before. These symptoms started 3 hours after receiving the first dose of the BNT162b2 mRNA vaccine intramuscularly. The most likely mechanism is that immune-mediated inflammatory demyelination of the cranial nerves is elicited [19,20].
Myocarditis induced by messenger RNA (mRNA) COVID-19 vaccines has been reported. Also reported was a potentially fatal disorder, Hemophagocytic Lymphohistiocytosis (HLH). It is manifested as fever, headache, nausea, progressive tachypnea, drowsy consciousness, mottling skin, jaundice, and hypotension. This is a severe hyperinflammatory syndrome induced by aberrantly activated macrophages and cytotoxic T cells. HLH could happen without a preexisting medical condition, or secondary to a malignant, infectious, or autoimmune/ autoinflammatory stimulus. An HLH patient is often critically ill with progressive multiple organ failure and requires intensive care. If untreated, the mortality could be 50%, in children [21].
Another reported rare complication following vaccine administration, Adult-Onset Still’s Disease (AOSD). It is a rare inflammatory disorder that usually affects young adults that characterized by high spike fever, transient evanescent skin rashes, arthralgia or arthritis, sore throat, leukocytosis, lymphadenopathy, and elevation of liver enzymes. Although the etiopathogenesis of this disease is not clear, evidence has shown that various mechanisms contribute to the pathogenesis, including genetic susceptibility, triggering factors, particularly infections, cytokine storm syndrome, and activation of the innate and adaptive immune system, which leads to the release of several inflammatory cytokines, thus causing inflammation in various organ systems [22].
Life-Course Vaccination Approach is a discovery in the field of immunology that vaccinations have on the immune system of individuals, increasing its plasticity and resilience recently defined as “immune fitness”.The rationale of the empirical approach of the vaccine has used the microorganisms or their toxins had to be attenuated or killed and injected into the vaccine to elicit an immune response in the recipient. This method enabled the development of vaccines that, while generally highly immunogenic, had several side effects due to their high reactogenicity. Tailored vaccinology is an innovative and interesting approach that includes synthetic biology and structural biology which allows the design of artificial molecules such as DNA and RNA, and the function of proteins. These approaches aim to design specific antigenic targets able to elicit a desired immunological response, opening the way to a new era of tailored vaccinology [23,24].
Result
The result of this research is summarised in the following tables (TABLE 1-6).
| Respondent’s Attitude towards COVID-19 Prevention Measures | Cluster: Anti-Vaccination Subjects (A) | Cluster: Followers of Vaccination (B) | Cluster: Those without Opinion (C) |
|---|---|---|---|
|
I generally do not believe in vaccination |
63% | 3.90% | 33.10% |
|
I do not have a problem with vaccination in general, but I do not trust COVID-19 vaccination |
61.10% | 5.60% | 33.30% |
|
It has not convinced me, nor am I against it yet, I think it is good to get |
49.10% | 9.90% | 40.90% |
|
Vaccinated against COVID- 19 |
14.60% | 76% | 9.30% |
|
I believe that vaccination against COVID-19 should be mandatory for adults who do not have medical contraindications |
5.30% | 73.70% | 21.10% |
|
N/A |
0% | 66.70% | 33.30% |
| Determinants of vaccine hesitancy | Variables |
|---|---|
|
Contextual influences |
Gender |
|
|
Age group |
|
|
Education |
|
|
Monthly household income |
|
|
Partial or total income loss during the pandemic |
|
|
Occupation |
|
|
Month of the questionnaire |
|
Individual influences |
Intention to take the flu vaccine |
|
|
Perception of the health status |
|
|
Number of comorbidities |
|
|
Having school-age children |
|
|
Frequency of agitation, sadness, or anxiety |
|
COVID-19 disease-specific |
Confidence in the health services response to COVID-19 |
|
|
Confidence in the health services response to non-COVID-19 |
|
|
Perception of the adequacy of measures implemented by the Government |
|
|
Self-perceived risk of getting COVID-19 infection |
|
|
Self-perceived risk of developing severe disease following COVID-19 infection |
|
COVID-19 vaccine-specific |
Confidence in the safety of the COVID-19 vaccines |
|
|
Confidence in the efficacy of the COVID-19 vaccines |
| Combo Post first dose | (n=42) BNT162b2 | (n=33) CoronaVac | (n=35) p2 | |
|---|---|---|---|---|
|
System reactions |
19 (45.2%) | 15 (45.5%) | 13 (37.1%) | 0.761 |
|
Fever |
2 (4.8%) | 1 (3.0%) | 0 (0) | 0.636 |
|
Chills |
0 (0) | 0 (0) | 2 (5.7%) | 0.187 |
|
Headache |
6 (14.3%) | 6 (18.2%) | 4 (11.4%) | 0.702 |
|
Tiredness |
12 (31.0%) | 11 (33.3%) | 9 (25.7%) | 0.807 |
|
Nausea |
1 (2.4%) | 1 (3.0%) | 3 (8.6%) | 0.516 |
|
Vomit |
0 (0) | 0 (0) | 0 (0) | - |
|
Diarrhea |
1 (2.4%) | 2 (6.1%) | 2 (5.7%) | 0.732 |
|
Muscle pain |
7 (16.7%) | 9 (27.3%) | 6 (17.1%) | 0.5 |
|
Joint pain |
1 (2.4%) | 4 (12.1%) | 2 (5.7%) | 0.255 |
|
Skin rash |
3 (7.1%) | 1 (3.0%) | 1 (2.9%) | 0.624 |
|
SAE |
0 (0) | 0 (0) | 0 (0) | - |
|
Local symptoms |
41 (97.6%) | 27 (81.8%) | 12 (34.3%) | <0.0001 |
|
Pain |
41 (97.6%) | 25 (75.8%) | 12 (34.3%) | <0.0001 |
|
Redness |
3 (7.1%) | 7 (21.2%) | 0 (0) | 0.005 |
|
Swelling |
6 (14.3%) | 12 (36.4%) | 0 (0) | <0.0001 |
|
Ecchymosis |
6 (14.3%) | 3 (9.1%) | 0 (0) | 0.2 |
|
Itching |
2 (4.8%) | 3 (9.1%) | 1 (2.9%) | 0.2 |
| n=918 | AEFV | Percentage (%) | p-Value | |
|---|---|---|---|---|
|
Bleeding |
||||
|
Adenovector |
733 | 37 | 5% | 0.33 |
|
mRNA |
98 | 5 | 5.1% | |
|
Inactivated vaccine |
57 | 6 | 10.5% | |
|
Live attenuated |
2 | 0 | 0% | |
|
Others |
28 | 3 | 10.7% | |
|
Seizure |
||||
|
Adenovector |
728 | 31 | 4.3% | 0.1 |
|
mRNA |
102 | 3 | 2.9% | |
|
Inactivated vaccine |
60 | 4 | 6.7% | |
|
Live attenuated |
2 | 0 | 0% | |
|
Others |
28 | 4 | 14.3% | |
|
Breathing difficulty |
||||
|
Adenovector |
750 | 19 | 2.5% | 0.9 |
|
mRNA |
100 | 3 | 3% | |
|
Inactivated vaccine |
61 | 2 | 3.3% | |
|
Live attenuated |
2 | 0 | 0% | |
|
Others |
30 | 5 | 16.7% | |
|
Hearing/Vision |
||||
|
Adenovector |
757 | 11 | 1.5% | 0.09 |
|
mRNA |
101 | 3 | 2.9% | |
|
Inactivated vaccine |
60 | 3 | 5% | |
|
Live attenuated |
2 | 0 | 0% | |
|
Others |
28 | 4 | 14.3% | |
|
Severe allergic reaction |
||||
|
Adenovector |
752 | 16 | 2.1% | 0.35 |
|
mRNA |
102 | 3 | 2.9% | |
|
Inactivated vaccine |
60 | 3 | 5% | |
|
Live attenuated |
2 | 0 | 0% | |
|
Others |
28 | 4 | 14.3% | |
| Frequency (n=969) | Percentage (%) | p-Value | |
|---|---|---|---|
|
Bleeding/Unusual weakness |
|||
| No | 918 | 94.7% | <0.0001 |
| Yes | 51 | 5.3% | |
|
Died after vaccination |
|||
| No | 920 | 94.9% | <0.0001 |
| *Yes | 49 | 5.1% | |
|
Seizure (convulsion) or high fever after hours or a few days |
|||
| No | 927 | 95.7% | <0.0001 |
| Yes | 42 | 4.3% | |
|
Breathing difficulty |
|||
| No | 943 | 97.3% | <0.0001 |
| Yes | 26 | 2.7% | |
|
Hearing/Vision problem |
|||
| No | 948 | 97.8% | <0.0001 |
| Yes | 21 | 2.2% | |
|
Clinical trial |
|||
| No | 622 | 64.2% | <0.0001 |
| Yes | 347 | 35.8% | |
*The deaths were accounted for by the healthcare workers that attended to the vaccinees with adverse events leading to deaths or the family members of the dead persons
| Frequency (n=969) | Percentage (%) | p-Value | |
|---|---|---|---|
|
Experienced uncommon signs including |
|
|
<0.0001 |
|
None |
651 | 67.1% |
|
|
Feeling dizzy |
116 | 11.9% | |
|
Decreased appetite |
62 | 6.4% | |
|
Excessive sweating |
41 | 4.2% | |
|
Abdominal pain |
32 | 3.3% | |
|
Itchy skin or rash |
28 | 2.9% | |
|
Enlarged lymph nodes |
23 | 2.4% | |
|
Menstrual disorder |
5 | 0.5% | |
|
Hunger |
4 | 0.4% | |
|
Increased libido |
2 | 0.2% | |
|
Experienced common signs including |
|
|
<0.0001 |
|
None |
508 | 52.4% |
|
|
Fever |
320 | 33% | |
|
Swelling, redness or a lump at the injection site |
176 | 18.2% | |
|
Flu-like symptoms such as high temperature, sore throat, runny nose, cough and chills |
117 | 12.1% | |
|
Being sick (vomiting) |
44 | 4.5 | |
|
Diarrhoea |
20 | 2.1% | |
|
Heaviness of the head |
2 | 0.2% | |
|
Bone ache |
1 | 0.1% | |
|
Lymph node enlargement |
1 | 0.1% | |
|
Experienced very common signs including |
|
|
<0.0001 |
|
None |
220 | 22.7% |
|
|
Feeling tired/fatigued |
388 | 40% | |
|
Tenderness, pain, warmth, itching or bruising where the injection was given |
380 | 39.2% | |
|
Headache |
363 | 37.5% | |
|
Generally feeling unwell |
339 | 34.9% | |
|
Chills or feeling feverish |
293 | 30.2% | |
|
Joint pain/muscle ache |
269 | 27.8% | |
|
Feeling sick/nausea |
115 | 11.9% | |
|
Deep sleep |
5 | 0.5% | |
|
Lymph in armpits |
3 | 0.3% | |
|
Mouth sores |
1 | 0.1% | |
|
Boil |
1 | 0.1% | |
|
Experienced lower sex drive |
1 | 0.1% | |
|
Diarrhoea |
1 | 0.1% | |
|
Ear pain |
1 | 0.1% | |
|
Chest pain |
1 | 0.1% | |
|
Vomiting |
1 | 0.1% | |
|
Blood (red) spot on left eye |
1 | 0.1% | |
|
Tender swollen tongue, loss of taste and appetite |
1 | 0.1% | |
|
Insomnia |
1 | 0.1% | |
|
Dry cough |
1 | 0.1% | |
|
Rhinitis |
1 | 0.1% | |
|
Numbness at neck and hand after 2nd dose for one night |
1 | 0.1% |
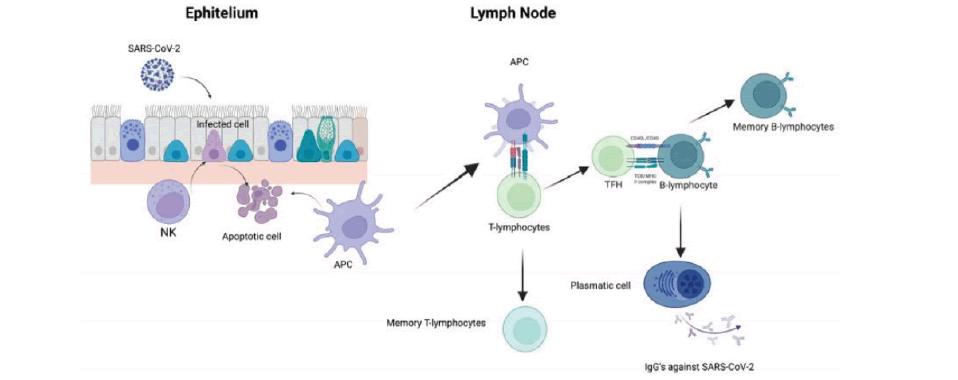
Infected cells might be recognized and eliminated by NK cells, followed by the recruitment of Dendritic Cells. DCs become APCs and migrate to the lymphatic node to present viral antigens to T-lymphocytes and generate an adaptive immune response composed of CD4+ T-lymphocytes. TFH interacts with B-lymphocytes to produce antibodies against the virus (FIGURE 1).
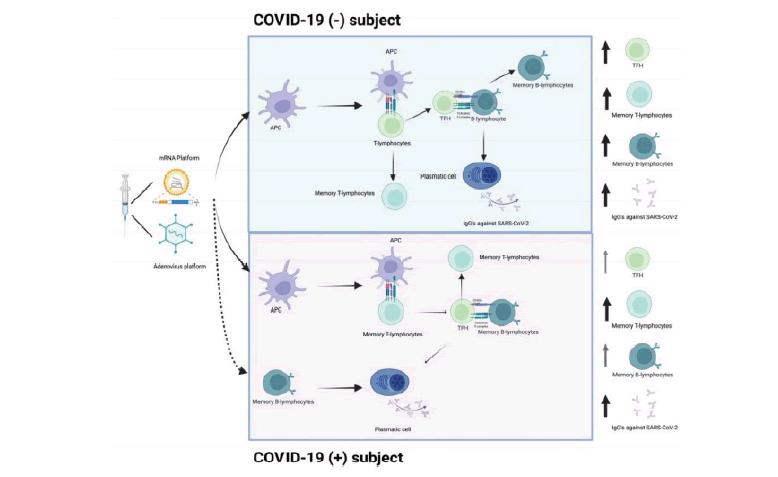
The vaccine induces the production of high levels of S protein, and adjuvants enhance the recruitment and differentiation of DCs into APCs. In COVID-19(−) subjects, APCs present the antigen to T-lymphocytes. TFH helps S protein-specific B-lymphocytes to differentiate into plasmatic cells and promote the production of IgGs against S protein. In contrast, in recovered COVID-19(+) subjects, SARS-CoV-2 specific memory T-lymphocytes, and memory B-lymphocytes developed after infection, could be activated by vaccine application and quickly and efficiently respond to antigen recall (FIGURE 2)
Discussion
COVID-19 virus is the most interesting pandemic in the last 3 years ago because of its life-threatening symptoms which increase entrance to intensive care and increase mortality rate. World health organization presents many recommendations regarding this pandemic, keep social distancing, use preventive hygienic measures, wear personal protective equipment and eat healthily. Vaccine is the magical invented tool to fight against the spread of covid 19 viruses. Incidence of immediate reaction following vaccination being reported. Despite the immediate reaction being mild and subjective, there is increased anxiety among the population regarding this vaccine especially as there are no definitive studies that can determine the predicted long-term side effects [25]. WHO shows some hesitancy regarding licensing the vaccines, the high rate of vaccine refusal may be partly due to the widespread fake news and conspiracy theories about vaccine safety and efficacy. Such information can cause fear and raise doubts about the origin and safety of vaccines, and consequently pose threats to the massive vaccination campaign so the success of a launch often depends on people’s willingness to accept any of them [26]. Decreasing the hesitancy towards COVID-19 vaccination will be the key to ending the pandemic and keeping endemic COVID-19 under control. WHO, now licensed the vaccine, as the vision is the benefits of using vaccines are more than the predicted risk. Despite this, the adverse event remains rare, and the overall risk of complications remains low. But, every effort is done to raise awareness among physicians about suspected side effects to help patients and diagnosis should be prompt for proper early management, to avoid potentially serious complications.
Conclusions
Vaccination is the most viable strategy to prevent or diminish the disease. Decreasing hesitancy towards COVID-19 vaccination will be key to ending the pandemic and keeping endemic COVID-19 under control. This hesitancy regarding vaccines is due to resultant adverse reactions. These reactions, which occur in a rare percentage of the vaccinated population, should not be a contraindication or a reason to avoid vaccination. The effect of the COVID-19 vaccine in patients with pre-existing inflammatory-immune-mediated rheumatic diseases found that the incidence of local and systemic adverse events, increased disease flares when compared with the primary series of vaccinations. As long as vaccines can act as triggers for extremely rare adverse effects, caution should be paid with the administration of additional vaccine doses in individuals who experience any sign or symptoms of adverse reaction. Physicians should be aware of all the most common, and less common adverse effects in patients after COVID-19 vaccination, and diagnosis should be prompt for proper early management, to avoid complications.
Recommendation
Highly professionally trained nurse staff on vaccine administration. It becomes necessary to critically evaluate patients’ previous medical histories and vaccine-associated allergies in detail. It is also important to monitor vaccinated persons for at least 30 min following COVID-19 vaccine administration to ensure that no immediate untoward events are observed.
Funding
The authors did not receive any financial support.
Conflict of Interest
No conflict of interest.
References
- Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 19(3), 141-154 (2021).[Google Scholar] [Crossref]
- Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N Engl J Med. 384, 1885-1898 (2021).[Google Scholar] [Crossref]
- Yang Y, Li W, Zhang Q, et al. Mental health services for older adults in China during the COVID-19 outbreak. Lancet Psychiatry. 7(4), e19 (2020).[Google Scholar] [Crossref]
- Xie J, Ding C, Li J, et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 92(10), 2004-2010 (2020).[Google Scholar] [Crossref]
- Peled Y, Ram E, Lavee J, et al. Third Dose of the BNT162b2 Vaccine in Heart Transplant Recipients: Immunogenicity and Clinical Experience. J Heart Lung Transplant. 41(2), 148-157 (2022).[Google Scholar] [Crossref]
- Rogliani P, Chetta A, Cazzola M, Calzetta L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines. 9(3), 227 (2021).[Google Scholar] [Crossref]
- Siewe B, Nipper AJ, Sohn H, Stapleton JT, Landay A. FcRL4 Expression Identifies a Pro-Inflammatory B Cell Subset in Viremic HIV-Infected Subjects. Front Immunol. 8, 1339 (2017).[Google Scholar] [Crossref]
- Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunedominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 5(48), 2992 (2020).[Google Scholar] [Crossref]
- World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification. 2019. [Google Scholar] [Crossref]
- MacDonald NE, Eskola J, Liang X, et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 33(34), 4161-4164 (2015).[Google Scholar] [Crossref]
- World Health Organization. Report of the SAGE Working Group on Vaccine Hesitancy. 2014.[Google Scholar] [Crossref]
- Spencer JP, Pawlowski RHT, Thomas S. Vaccine Adverse Events: Separating Myth from Reality. Am Fam Physician. 95(12), 786-794 (2017).[Google Scholar] [Crossref]
- CDC. Selected Adverse Events Reported after COVID-19 Vaccination. 2022 [Google Scholar] [Crossref]
- CDC. What to Do if You Had an Allergic Reaction after Getting a COVID-19 Vaccine. 2022. [Google Scholar] [Crossref]
- Characteristics of SARS-CoV-2 and COVID-19
- Sampath V, Rabinowitz G, Shah M, et al. Vaccines and allergic reactions: The past, the current COVID-19 pandemic and future perspectives. Allergy. 76(6), 1640-1660 (2021).[Google Scholar] [Crossref]
- Hua W, Izurieta HS, Slade B, et al. Kawasaki Disease after Vaccination: Reports to the vaccine adverse event reporting system 1990-2007. Pediatr Infect Dis J. 28(11), 943-947 (2009).[Google Scholar] [Crossref]
- Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis With Polyangiitis. Arthritis Rheumatol. 74(3), 386-392 (2022).[Google Scholar] [Crossref]
- Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: A case series and nested case-control study. Lancet Infect Dis. 22(1), 64-72 (2021).[Google Scholar] [Crossref]
- Finsterer J, Scorza FA, Scorza CA. Post SARS-CoV-2 vaccination Guillain-Barre syndrome in 19 patients. Clinics. 76, e3286 (2021).[Google Scholar] [Crossref]
- Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic lymphohistiocytosis: A review inspired by the COVID-19 pandemic. Rheumatol Int. 41(1), 7-18 (2021).[Google Scholar] [Crossref]
- Mitrovic S, Fautrel B. Clinical Phenotypes of Adult-Onset Still’s Disease: New Insights from Pathophysiology and Literature Findings. J Clin Med. 10(12), 2633 (2021).[Google Scholar] [Crossref]
- De Gregorio E, Rappuoli R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat Rev Immunol. 14(7), 505-514 (2014).[Google Scholar] [Crossref]
- Lurie N, Saville M, Hatchett R, Halton J. Developing COVID-19 Vaccines at Pandemic Speed. N Engl J Med. 382(21), 1969-1973 (2020).[Google Scholar] [Crossref]
- Blumenthal KG, Robinson LB, Camargo CA, Shenoy ES. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA. 325(15), 1562-1565 (2021).[Google Scholar] [Crossref]
- Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 5(3), 337-348 (2021).[Google Scholar] [Crossref]