Case Report - (2023) Volume 6, Issue 1
Lead Identification against Polycystic Ovary Syndrome using Molecular Docking Approach
Kavita Pandey*, Priyankal Sharma, Ananya Chavan and Priyanka PanditReceived: Feb 09, 2023, Manuscript No. amdhs-23-89097; Editor assigned: Feb 14, 2023, Pre QC No. amdhs-23-89097 (PQ); Reviewed: Feb 23, 2023, QC No. amdhs-23-89097 (Q); Revised: Feb 25, 2023, Manuscript No. amdhs-23-89097 (R); Published: Mar 10, 2023, DOI: 10.5530/amdhs.2023.1.3
Abstract
Polycystic Ovary Syndrome (PCOS) remains a major health problem in women, characterized by hyperinsulinemia, hyperandrogenism, menstrual irregularities, and long-term metabolic disturbances. Ovarian steroidogenesis is one of the key signalling pathways for follicular development. Due to the overexpression of the key enzymes (CYP 17A1, CYP 11A1, CYP 1A1, CYP 19A1, STAR and HSD17B1) present in this pathway, androgen is converted more efficiently to testosterone, causing hyperandrogenism and hyperinsulinemia. In this study, virtual screening of 203 phytochemicals collected from 44 plants were used as ligands to identify potent inhibitors for each of the promising targets of PCOS. Subsequently, a multitargeted docking operation was carried out, which rendered the 22 best docking poses. In-silico ADME and drug-likeness prediction of 9 of these 22 phytochemicals showed good pharmacokinetic properties, having high gastrointestinal absorption, blood-brain barrier permeability, and less toxic. Six phytochemicals: Chlorogenin, Hispaglabridin B, Ruscogenin, Glabridin, Degulin, and Diosgenin were evaluated as the best potential inhibitors for HSD17B1, CYP11A1, STAR, CYP1A1 CYP19A1 and CYP17A1, PCOS proteins, respectively. This study is believed to aid in the identification and future lead optimization for the natural treatment of PCOS.
https://blogum.blogaaja.fi/
https://blogum-1.jimdosite.com/
https://blogummm.edublogs.org/
https://blogummm.websites.co.in/
https://blogum18.wordpress.com/
https://benim-blogum.jigsy.com/
https://fuiegs-symbeaurds-build.yolasite.com/
https://blogum-03.webselfsite.net/
https://blogummm.mystrikingly.com/
https://blogum.splashthat.com/
https://blogum3.webnode.com.tr/
https://blogum.odoo.com/
https://blogum.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/enrollments
https://blogum.estranky.cz/
https://653ba4fbb538c.site123.me/
https://blogum12m.blogspot.com/
https://blogum.hashnode.dev/
https://whiteseoturkey1.wixsite.com/blogum
https://sites.google.com/view/blogummm/
https://codepen.io/blogum
https://blogumm.livejournal.com/
https://wakelet.com/@blogum82816
https://www.homify.com/users/9538383/blogum
https://lessons.drawspace.com/profile/323613/blogum
https://my.desktopnexus.com/blogum/
https://writeupcafe.com/profile/BLOGUM/
https://www.pearltrees.com/blogum
https://www.easyfie.com/blogum
https://pharmahub.org/members/27615/profile
https://www.zupyak.com/u/blogum/posts
https://www.metroflog.co/blogum
https://www.fuzia.com/fz/blogum-blogum
https://tr.pinterest.com/blogum12/
https://my.getjealous.com/blogum
https://micro.blog/blogum
https://www.tumblr.com/blogummm
https://hub.docker.com/u/blogum
https://fire.blogfree.net/?act=Profile&MID=1342323
https://blogum.pixnet.net/blog
https://www.threadless.com/@blogum/activity
https://blogum.neocities.org/
https://blogum12.amebaownd.com/
https://teletype.in/@blogum
https://ubl.xml.org/users/blogum
https://educatorpages.com/site/blogum/
https://blogum.onlc.fr/
Keywords
PCOS, Ovarian steroidogenesis, Phytochemicals, Molecular Docking, Hyperandrogenism, Hyperinsulinemia
Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrinopathies and cause of anovulatory infertility in women [1]. When completely expressed, PCOS patients have ovulatory dysfunction (oligomenorrhea, increased risk of infertility) and androgen excess (hirsutism and insulin resistance with or without obesity) [2,3]. Women with PCOS have a higher prevalence of risk factors for type 2 diabetes and cardiovascular diseases [4,5].
One of the primary signalling routes for follicular growth is ovarian steroidogenesis [6]. Women with PCOS have aberrant ovarian steroidogenesis and exhibit hyperandrogenemia. Furthermore, insulin resistance and hyperinsulinemia affect 50%-70% of PCOS women [4]. Hyperinsulinemia has been related to high testosterone levels and anovulation. Insulin interacts with gonadotrophins and affects steroidogenesis by acting on the ovary via its receptor. Insulin's exact purpose and the chemical mechanisms that cause it are still unknown [7]. Because critical ovarian steroidogenesis pathway enzymes (CYP17A1, CYP11A1, CYP1A1, CYP19A1, STAR, and HSD17B1) are overexpressed, androgen is converted to testosterone more efficiently, leading to hyperandrogenism [9-13].
Virtual screening is a vital tool in in-silico screening of drug candidates [8]. Virtual screening of 203 phytochemicals collected from 44 plants based on their anti-androgenic and anti-diabetic properties were used as ligands in this study to identify potent inhibitors for each of the above mentioned target proteins using the blind docking method. SwissADME was also used to assess the pharmacological properties of the phytochemicals with the lowest binding affinities in order to forecast their appropriateness as a PCOS treatment agent [15].
Material and Methods
The data of ovarian steroidogenesis pathway and respective target proteins were collected from PCOSKB database. On the other hand phytochemicals were shortlisted via comprehensive literature survey based on their antiandrogenic and anti-diabetic properties.
■ Protein preparation
Three-dimensional structures of the target shortlisted proteins were obtained using Protein Data Bank. The native ligand was separated from the protein, polar hydrogen atoms were added, water molecules and heteroatoms were removed, and the 3D structures were validated using the SAVES server (TABLE 1). The file were saved in PDBQT format for further examination after Kollman charges were applied in the PyRx 0.8 virtual screening tool.
TABLE 1. Target Proteins associated with PCOS for the docking study
| Sr. No | Protein name | PDB ID | PDB structure |
|---|---|---|---|
| 1 | HSD17B1 | 1JTV |  |
| 2 | CYP11A1 | 3N9Y |  |
| 3 | STAR | 3P0L |  |
| 4 | CYP1A1 | 4I8V |  |
| 5 | CYP19A1 | 5JKV |  |
| 6 | CYP17A1 | 6WR1 |  |
■ Ligand preparation
Through intensive literature survey, 203 ligands (phytochemicals) shortlisted for their therapeutic potential against PCOS. The 3D conformers of these ligands were derived from PubChem database. The compounds were imported into Open Babel within PyRx 0.8 virtual screening tool and subjected to energy minimization and then transformed to PDBQT format for further analysis.
■ Virtual screening
Structure- based virtual screening applying molecular docking was performed using the AutoDock Vina tool compiled in PyRx 0.8 virtual screening software. The structures of the selected target proteins crystal previously disclosed were used as the macromolecule (receptor). The grid box encompassed the entire stretch of crystal implementing randomized docking protocol. The docking was then conducted with an exhaustiveness of 8. The results were assessed by sorting the various complexes according to the estimated binding energies (kcal/mol). The listed in 3D structure of the target protein HSD17B1 related with PCOS was obtained from Protein Data Bank. The native ligands were separated from protein, polar hydrogen atoms were added, water molecules and heteroatoms were removed and the 3D structure was validated using the SAVES server. The file was saved in PDBQT format for further examination after Kollman charges were applied in the PyRx 0.8 virtual screening tool.
■ Pharmacokinetic properties and drug likeliness
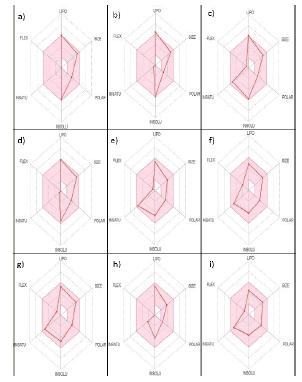
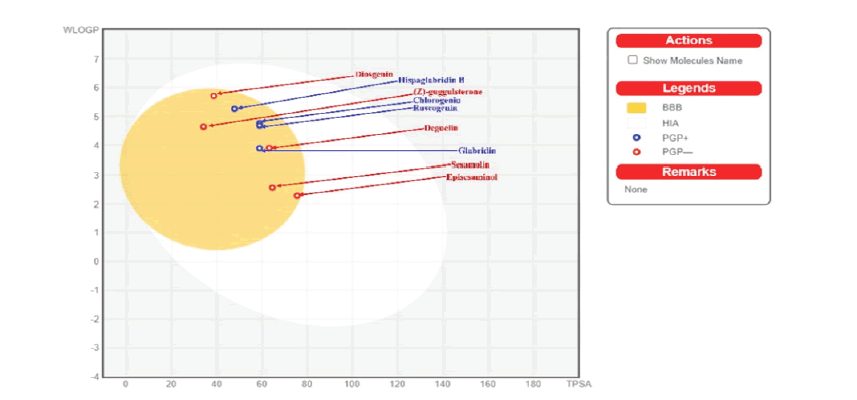
The binding affinity of inhibitors for their target proteins does not guarantee their usefulness as potential medicines in a biological system. The ADME investigations help in determining the drug-likeness of inhibitors in biological systems. As a result, its utilisation is required. To determine the pharmacokinetics of ligands and their suitability in a biological system, Swiss ADME was used to run Lipinski's Rule of 5 for the 22 phytochemicals with the best poses towards the target proteins (TABLE 2), Bioavailability Radar (FIGURE 1), and Egan's BOILED-Egg approach (FIGURE 2). Finally, the best docked postures with phytochemicals that had high pharmacokinetic properties were visualized in BIOVIA Discovery Studio Visualizer (TABLE 3 And TABLE 4).
TABLE 2. Molecular binding affinities of the best docking poses
| Sr. No. | Protein name (PDB ID) | Phytochemicals | Binding Affinity (kcal/mol) |
|---|---|---|---|
| 1 | HSD17B1 (1JTV) | Sarsasapogenin | -11.7 |
| Chlorogenin | -11.1 | ||
| Isoadiantone | -10.9 | ||
| 2 | CYP11A1 (3N9Y) | Hispaglabridin B | -11.6 |
| Ruscogenin | -11.2 | ||
| Taraxasterol acetate | -11.2 | ||
| 3 | STAR (3P0L) | Taraxasterol | -9.9 |
| 4 | CYP1A1 (4I8V) | Pongaglabol | -11.7 |
| Lanceolatin B | -11.6 | ||
| Purpurin | -11.4 | ||
| Glabridin | -11.1 | ||
| Quercetin-3-glucuronide | -11 | ||
| Sesamolin | -11 | ||
| 5 | CYP19A1 (5JKV) | Rutin | -10 |
| Miquelianin | -9.9 | ||
| Deguelin | -9.8 | ||
| (Z)-guggulsterone | -9.6 | ||
| (-)-Epigallocatechin-gallate | -9.6 | ||
| Maximaisoflavone c | -9.4 | ||
| Episesaminol | -9.4 | ||
| 6 | CYP17A1 (6WR1) | Diosgenin | -10.9 |
| Stigmasterol | -10.6 |
TABLE 3. Assessment of drug likeliness and pharmacokinetic properties of ligands (phytochemicals)
| Lipinski’s Rule of Five | |||||||
|---|---|---|---|---|---|---|---|
| Sr. No. | Phytochemical | Mol. Wt. (g/mol) | Lipophilicity (MLogP) | H Bond Donors | H Bond Acceptors | Rule Voilations | Follow R05 |
| 1 |
Sarsasapogenin |
416.64 | 5.08 | 1 | 3 | 1 | Y |
| 2 |
Chlorogenin |
432.64 | 4.23 | 2 | 4 | 1 | Y |
| 3 |
Diosgenin |
414.62 | 4.94 | 1 | 3 | 1 | Y |
| 4 |
Isoadiantone |
412.69 | 6.73 | 0 | 1 | 1 | Y |
| 5 |
Hispaglabridin B |
390.47 | 3.73 | 1 | 4 | 0 | Y |
| 6 |
Ruscogenin |
430.62 | 4.09 | 2 | 4 | 0 | Y |
| 7 |
Taraxasterol acetate |
468.75 | 7.08 | 0 | 2 | 1 | Y |
| 8 |
Taraxasterol |
426.72 | 6.92 | 1 | 1 | 1 | |
| 9 |
Pongaglabol |
278.26 | 1.43 | 1 | 4 | 0 | Y |
| 10 |
Lanceolatin B |
262.26 | 2.01 | 0 | 3 | 0 | Y |
| 11 |
Purpurin |
256.21 | 0.11 | 3 | 5 | 0 | Y |
| 12 |
Glabridin |
324.37 | 2.73 | 2 | 4 | 0 | Y |
| 13 |
Quercetin-3-glucuronide |
478.36 | -2.6 | 8 | 13 | 2 | N |
| 14 |
Sesamolin |
370.35 | 1.85 | 0 | 7 | 0 | Y |
| 15 |
Stigmasterol |
412.69 | 6.62 | 1 | 1 | 1 | Y |
| 16 |
Rutin |
610.52 | -3.89 | 10 | 16 | 3 | N |
| 17 |
Miquelianin |
478.36 | -2.6 | 8 | 13 | 2 | N |
| 18 |
Deguelin |
394.42 | 1.93 | 0 | 6 | 0 | Y |
| 19 |
(Z)-guggulsterone |
312.45 | 3.86 | 0 | 2 | 0 | Y |
| 20 |
(-)-Epigallocatechin-gallate |
458.37 | -0.44 | 8 | 11 | 2 | N |
| 21 |
Maximaisoflavone c |
380.39 | 1.94 | 0 | 6 | 0 | Y |
| 22 |
Episesaminol |
370.35 | 1.45 | 1 | 7 | 0 | Y |
TABLE 4. Lead phytochemicals showing best binding affinities towards the target proteins selected for visualization.
| Sr no | Phytochemicals | Target Protein | Binding affinity (kcal/mol) |
|---|---|---|---|
| 1 | Chlorogenin | HSD17B1 | -11.1 |
| 2 | Diosgenin | CYP17A1 | -10.9 |
| 3 | Hispaglabridin B | CYP11A1 | -11.6 |
| 4 | Ruscogenin | STAR | -9.8 |
| 5 | Glabridin | CYP1A1 | -11.1 |
| 6 | Deguelin | CYP19A1 | -9.8 |
Results and Discussion
■ Molecular docking analysis
PCOS is characterized by the growth of cysts in the ovary and affects the ovulation phase of reproductive-aged women between the ages of 12-51. Synthetic medications are used to treat PCOS, which causes complications. As a result, alternative treatments that have less adverse effects are required. Ayurveda and natural substances have traditionally played an important part in medical treatment and are now widely used to treat a wide spectrum of conditions. As a result, natural chemicals can be exploited as a substitute for current medications. In current study the ovarian steroidogenesis pathway and its target proteins were selected and obtained from the PCOSKB database. The target protein was docked with 203 phytochemicals using molecular docking tool PyRx. Molecular docking is a computational approach that evaluates the optimal binding conformation of a ligand with the active site of a target [16]. The lower the docking score, the better the docking affinity and the interaction of protein and ligand were ranked based on binding affinity [8,20]. When the ligand involved efficiently binds with the active pockets of the target, which determines the significance and sensitivity of binding affinity values. TABLE 2 demonstrates the binding affinities of the best 22 poses of the 203 phytochemicals with the HSD17B1, CYP11A1, STAR, CYP1A1 CYP19A1 and CYP17A1. PCOS protein targets, respectively. The binding affinity of complexes was found to be between -9.2 kcal/mol and -11.7 kcal/mol, indicating their substantial potency and can be a possible inhibitor of targeted enzyme proteins.
■ In Silico Pharmacokinetic Predictions
ADME (absorption, distribution, metabolism, and excretion) including drug-likeness analysis, are crucial in drug development since they help to determine if inhibitors can be delivered to biological systems [18,19]. Lipinski's rule of 5, was developed by Christopher A. Lipinski in 1997 to determine if an inhibitor with specific biological and pharmacological features would be an orally active medication in the human body [18]. If 2 or more of these thresholds are fulfilled, an inhibitor can be orally absorbed; Molecular Weight (MW) of molecule <500, octanol/water partition coefficient (iLOGP)≤ 5, number of Hydrogen Bond Acceptors (nHBA) ≤10, number of Hydrogen Bond Donors (nHBD)≤5. From the output of some ADME and drug-likeness properties shown in TABLE 3, it was discovered that 18 molecules have zero or one violation of the Lipinski's rule, whereas Quercetin-3-glucuronide, Rutin, Miquelianin, and Epigallocatechin-gallate have 2-3 violations and were therefore evicted. Furthermore, the bioavailability radar uses six physicochemical qualities to quickly assess a molecule's drug-likeness: saturation, lipophilicity, polarity, size, solubility, and flexibility [15]. In this current study out of the 18 phytochemicals filtered by Lipinski’s rule FIGURE 1 (a-i), shows the bioavailability radars of 9 phytochemicals whose radar plots fall under the pink area which represent the optimal range for each properties (flexibility: no more than 9 rotatable bonds , size:, polarity: TPSA between 20 and 130Å2, MW between 150 g/mol and 500g/mol , Saturation: fraction of carbons in the sp3 hybridization not less than 0.25, solubility: log S not higher than 6 and lipophilicity: XLOGP3 between −0.7 and +5.0.)
All compounds depicted as dots in FIGURE 2, have high gastrointestinal absorption and are non-substrate (PGP) to P-glycoprotein in red, except Chlorogenin, Hispaglabridin B, Ruscogenin, and Glabridin in blue, which are projected to be actively effluxed by P-gp (PGP+). The SwissADME online webserver also developed the Brain or Intestinal estimated permeation predictive model (BOILED Egg), also known as Egan egg graph of the 9 compounds, which gave a proper graphical representation of the chemicals' absorption in the brain and gastrointestinal tract [19]. The graph molecules in the yolk area (yellow) are predicted to pass through the blood-brain barrier inactively. The molecules are expected to be orally accessible (low polarity and flexibility), less poisonous, and absorb well.
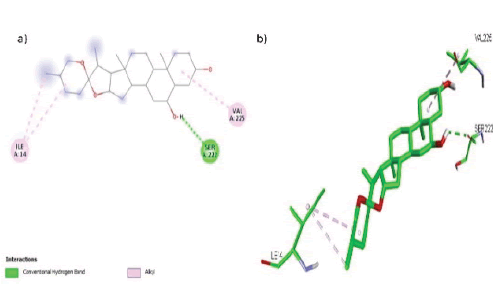
Six of the nine phytochemicals were chosen for visualization in the BIOVIA Discovery Studio Visualizer (TABLE 4). In the active pocket of the target HSD17B1, chlorogenin formed 3 hydrophobic contacts (Alkyl) with two amino acid residues (Ile 14, Val 255) and a hydrogen bond with Ser 222, as illustrated in FIGURE 3 (a, b), which shows the key amino acid residues involved in interactions with HSD17B1.
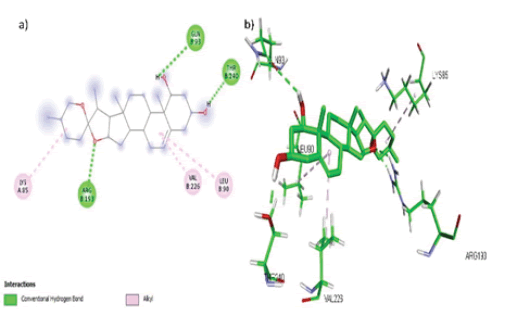
Diosgenin formed 6 hydrophobic interactions (Alkyl) with amino acids Arg 239, Ile 205, Ala 302, Ala 113, Ala 367 and Ile 371 while 2 hydrogen bonds with amino acid residues Gly 301 and Cys 402 in the active site of the target CYP17A1 as shown in FIGURE 4 (a, b).
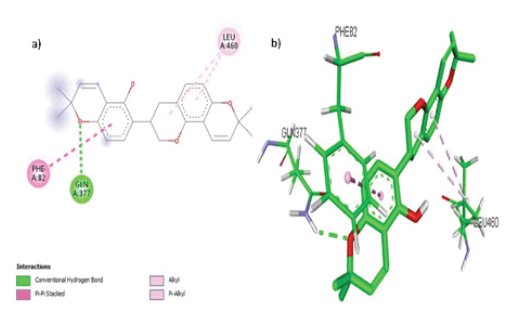
Hispaglabridin B formed 3 hydrophobic interactions (Alkyl and Pi-Alkyl) with 1 amino acid residue (Leu 460), a Pi-Pi Stacked bond with amino acid Phe 82 and a hydrogen bond with Gly 377 in the active pocket of the target CYP11A1 as shown in FIGURE 5 (a, b).
Ruscogenin formed 3 hydrophobic interactions (Alkyl) with amino acids Lys 85, Val 226 and Leu 90 while 3 hydrogen bonds with amino acid residues Gly 93, Arg 193 and Thre 240 in the active site of the target STAR as shown in FIGURE 6 (a, b).
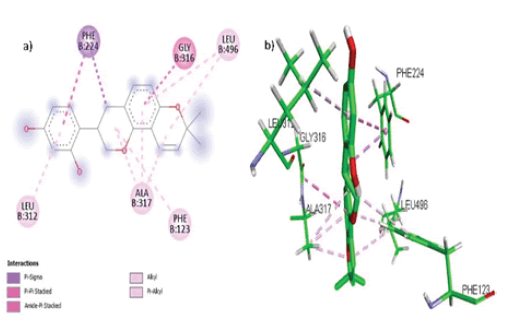
Glabridin formed 7 hydrophobic interactions (pi-Alkyl, Alkyl) with 4 amino acids Leu 496, Leu 312, Ala 317, Phe 123 while Amide-Pi Stacked, pi-Sigma, and pi-pi Stacked bonds with 2 amino acids Phe 224 and Gly 316 in the active site of the target CYP1A1 as shown in FIGURE 7 (a, b).
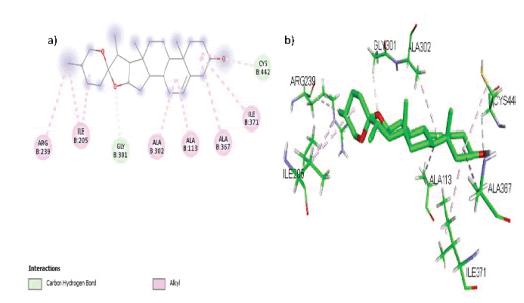
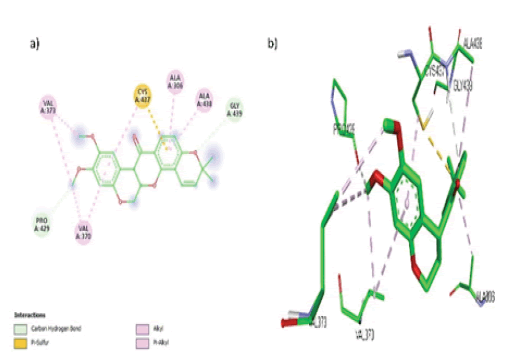
In the active region of the target CYP19A1, Deguelin formed 7 hydrophobic contacts (Alkyl, Pi-Alkyl) with amino acids Val 373, Val 370, Ala 306, and Ala 438, a Pi-Sulphur interaction with Cys 437, and 2 hydrogen bonds with amino acid residues Pro 429 and Gly 439, as shown in FIGURE 8 (a, b).
Conclusions
The theoretical evaluation of phytochemical binding affinities (kcal/mol) with 6 PCOS targets was carried out in this study to evaluate their potency and identify potential lead compounds. The molecular docking studies indicated a good docking score of -9.2 kcal/mol to -11.7 kcal/mol, indicating that the compounds can bind more tightly with the targets' active sites. Chlorogenin, Diosgenin, Hispaglabridin B, Ruscogenin, Glabridin, and Deguelin had the best binding postures towards HSD17B1, CYP11A1, STAR, CYP1A1, CYP19A1, and CYP17A1 targets. Furthermore, the ADME and drug-likeness properties revealed that the nine phytochemicals Chlorogenin, Diosgenin, Hispaglabridin B, Ruscogenin, Glabridin, Sesamolin, Deguelin, (Z)-guggulsterone, and Episesaminol have good pharmacokinetic properties, indicating that they are orally bioavailable, less toxic, and have good absorption. Furthermore, the increased amount of H-bonds generated by the phytochemicals-interacting affords structural insight to support the notion that it was able to engage closely with the targeted enzyme's active region. Computational screening for novel herbal inhibitors (phytochemicals) could be useful in producing commercial formulations for better Polycystic Ovary Syndrome (POS) therapy, either alone or in combination with other better phytochemicals, or in combination with integrated approaches.
References
- Barthelmess EK, Naz RK. Polycystic ovary syndrome: current status and future perspective. Front Biosci. 6(1), 104 (2014).
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 38(9), 1165-1174 (1989).
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 352(12), 1223-1236 (2005).
- Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 91(2), 492-497 (2006).
- Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of Adolescent Polycystic Ovary Syndrome to Parental Metabolic Syndrome. J Clin Endocrinol Metab. 91(4), 1275-1283 (2006).
- Tucker EJ, Jaillard S, Sinclair AH. Genetics and Genomics of Primary Ovarian Insufficiency. Hum Reprod Prenat Genet. 427-445 (2019).
- Diamanti-Kandarakis E, Argyrakopoulou G, Economou F, Kandaraki E, Koutsilieris M. Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS). J Steroid Biochem Mol Biol. 109(3-5), 242-246 (2008).
- Belani M, Deo A, Shah P, et al. Differential insulin and steroidogenic signaling in insulin resistant and non-insulin resistant human luteinized granulosa cells-A study in PCOS patients. J Steroid Biochem Mol Biol. 178, 283-292 (2018).
- Joseph S, Barai RS, Bhujbalrao R, Idicula-Thomas S. PCOSKB: A knowledgebase on genes, diseases, ontology terms and biochemical pathways associated with polycystic ovary syndrome. Nucleic Acids Res. 44(D1), D1032-D1035 (2016).
- Lecke SB, Morsch DM, Spritzer PM. CYP19 gene expression in subcutaneous adipose tissue is associated with blood pressure in women with polycystic ovary syndrome. Steroids. 76(12), 1383-1388 (2011).
- Pusalkar M, Meherji P, Gokral J, Chinnaraj S, Maitra A. CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil Steril. 92(2), 653-659 (2009).
- Sander VA, Hapon MB, Sícaro L, et al. (2011). Alterations of folliculogenesis in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 124(1-2), 58-64 (2011).
- Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss III JF. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol. 63(1), 51-60 (2004).
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 7(1), 1-13 (2017).
- Singh SP, Konwar BK. Molecular docking studies of quercetin and its analogues against human inducible nitric oxide synthase. Springer Plus. 1(1), 1-10 (2012).
- Pandey K, Upadhyay R, Gadani M. Effect of a Proprietary Arctostaphylous uva-ursi Standardized Extract (UvaZen-VArb™) on Melanin Regulation and Skin Applications-An In-silico Molecular Docking Study. Eur J Med Plants. 32(10), 12-22 (2021).
- Attique SA, Hassan M, Usman M. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int J Environ Res Public Health. 16(6), 923 (2019).
- Umar AB, Uzairu A, Shallangwa GA, Uba S. Design of potential anti-melanoma agents against SK-MEL-5 cell line using QSAR modeling and molecular docking methods. SN Appl Sci. 2(5), 1-18 (2020).
- Daina A, Zoete V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 11(11), 1117-1121 (2016).